Abstract
Background
Ponatinib, a third-generation BCR-ABL1 tyrosine kinase inhibitor (TKI), + hyper-CVAD showed remarkable activity against Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL), and may be superior to chemotherapy + earlier generation TKIs in terms of depth of remission, event-free survival (EFS), and overall survival (OS). However, this regimen's efficacy and tolerability have yet to be externally validated. Here, we summarize our real-world experience with ponatinib + hyper-CVAD for untreated Ph+ ALL and other Ph+ acute or blast phase leukemias.
Methods
We retrospectively analyzed all adults treated at the University of California, Davis (UCD) from March 2012 to May 2021 with ponatinib + hyper-CVAD upfront. The primary endpoints were 3-year OS and EFS. Secondary endpoints were complete molecular response (CMR), measurable residual disease (MRD) negativity by multiparameter flow cytometry (MFC), complete cytogenetic response (CCyR) rates, and adverse events (AEs). Time to event analyses were done via the Kaplan-Meier method. Patients alive were censored at their last follow-up date. Patients undergoing allogeneic hematopoietic cell transplant (HCT) after 6 months of achieving complete remission (CR) were censored at the time of HCT for the landmark analysis. Patients with missing data were excluded from the response analyses.
Results
We identified 13 Ph+ ALL patients who received ponatinib + hyper-CVAD for initial induction. The baseline characteristics for the Ph+ ALL patients are summarized in Table 1. The median follow-up was 16 months. The median number of hyper-CVAD cycles completed was 8 (range, 1-8) with ponatinib. Two patients proceeded to HCT in CR1, one at 3.5 months after starting induction, and due to difficulty controlling the patient's concurrent multiple myeloma prior to HCT and recovery from anti-neoplastic therapy, the second was delayed to 46 months after starting induction.
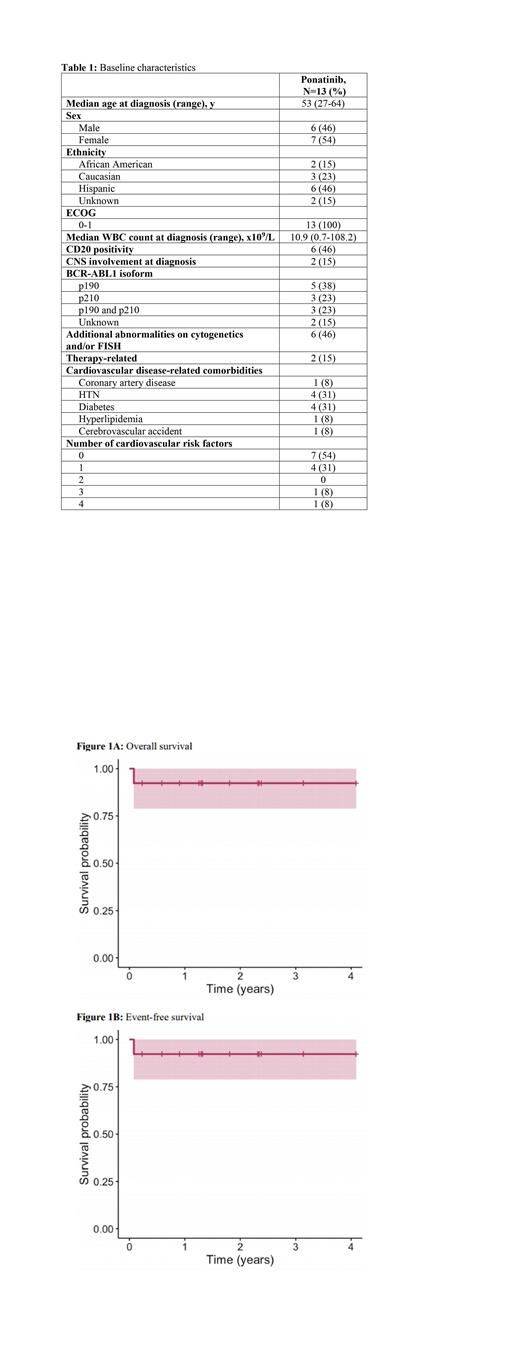
The 3-year OS and EFS with ponatinib + hyper-CVAD were each 92% (95% confidence interval, 78.9-100) (Figure 1). Landmark analysis completed 6 months following CR showed a 3-year OS of 100% in patients treated with ponatinib + hyper-CVAD without HCT in first CR (CR1). The CMR, CCyR, and MRD-negativity by MFC rates with ponatinib were all 92.3% (12/13). The median time to CMR, CCyR, and MRD-negativity by MFC were 51 days, 22 days, and 53 days, respectively.
Notable AEs with ponatinib include neutropenic fever (92%), bacterial infection (69%), transaminitis (38%), venous thromboembolism (31%), invasive fungal infection (15%), hemorrhage (15%), cerebrovascular accident (CVA) (15%), and tumor lysis syndrome (8%). One patient died during induction with ponatinib due to a bacterial infection. Two patients switched to a different TKI due to a CVA after 4 and 24 months. Only 2 patients did not complete 8 cycles of hyper-CVAD, due to death during induction (n=1) and proceeding to HCT after 3 cycles (n=1).
As for similar Ph+ leukemias, 3 chronic myeloid leukemia with lymphoid blast crisis (CML-LBC), 1 CML with mixed phenotype blast crisis (CML-MPBC), and 1 with mixed phenotype acute leukemia (MPAL) were treated with ponatinib + hyper-CVAD. The MPAL patient achieved CMR within 55 days, while the CML-MPBC and 2 CML-LBC patients achieved CMR after HCT. The third CML-LBC patient is in CR with ongoing treatment. After median follow-up of 25 months, all 5 were alive, and only the MPAL patient relapsed 28 months after starting treatment and 1 year after HCT.
Conclusion
To our knowledge, this is the first report externally validating the efficacy and tolerability of ponatinib + hyper-CVAD for Ph+ ALL. We also show the feasibility of using this regimen in patients with Ph+ CML-LBC, CML-MPBC and MPAL. Despite the small sample size and retrospective nature, our study supports existing data demonstrating that this regimen challenges both the designation of Ph+ ALL as a high-risk disease and the trend to transplant in CR1. Our findings support that ponatinib + hyper-CVAD should be considered a standard of care for Ph+ ALL.
Kaesberg: Incyte: Speakers Bureau. Rosenberg: Takeda, Janssen: Speakers Bureau. Abedi: BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Speakers Bureau; Seattle Genetics: Speakers Bureau. Tuscano: Genentech, Pharamcyclics, Abbvie, BMS, Acrotech, Seattle Genetics, Takeda: Research Funding. Jonas: AbbVie, BMS, Genentech, GlycoMimetics, Jazz, Pfizer, Takeda, Treadwell: Consultancy; 47, AbbVie, Accelerated Medical Diagnostics, Amgen, AROG, Celgene, Daiichi Sankyo, F. Hoffmann-La Roche, Forma, Genentech/Roche, Gilead, GlycoMimetics, Hanmi, Immune-Onc, Incyte, Jazz, Loxo Oncology, Pfizer, Pharmacyclics, Sigma Tau, Treadwell: Research Funding; AbbVie: Other: Travel reimbursement.
Ponatinib is approved for Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ALL) that is resistant or intolerant to prior tyrosine kinase inhibitor therapy. In this study, we described outcomes with ponatinib in combination with hyperCVAD in the frontline setting, which is off-label.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal